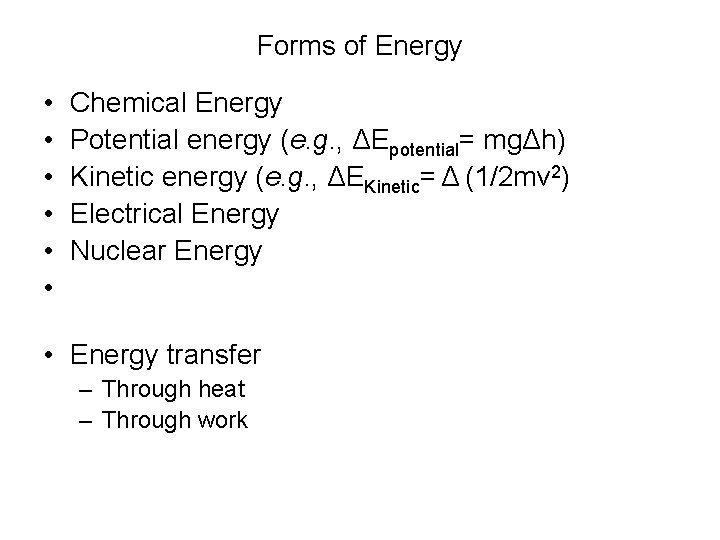
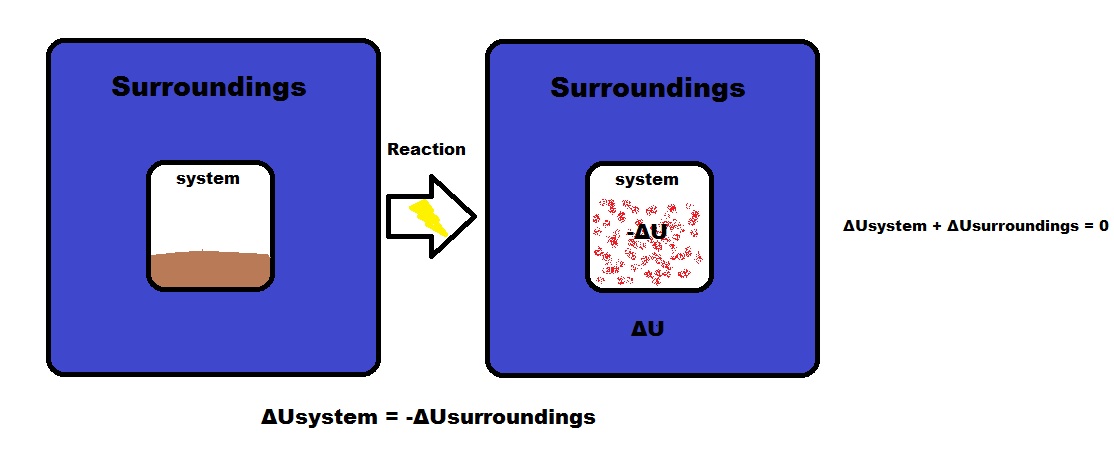
Conversely, the energy of an electric current can be used to bring about many chemical reactions that do not occur spontaneously A process involving the direct conversion of chemical energy when suitably organized constitutes an electrical cell A process whereby electrical energy is converted directly into chemical energy is one ofChemical Potential Energy Chemical energy occurs when energy is released during a chemical reaction Potential chemical energy is the energy stored in the bonds of these chemicals, ready to react with another chemicalThe energy from macronutrients comes from their chemical bonds This chemical energy is converted into cellular energy that can be utilized to perform work, allowing cells to conduct their basic functions Although vitamins also have energy in their chemical bonds, our bodies do not make the enzymes to break these bonds and release this energy

Chemical Energy Explained Youtube
What is an example of chemical energy
What is an example of chemical energy-Energy Idea for Mars Yields a Clue for Powering Data Centers Credit SUNNYVALE, Calif — As a scientist working for NASA in the 1990s, K R Sridhar developed a contraption that could useChemical energy from a battery is a potential form of energy, elastic energy in a stretched rubber band is a form of potential energy, but the most commonly referred to form of potential energy in physics is that of gravitational potential energy This is energy that is




Work Power Energy Learning Outcomes Define Work Energy
ENERGY VALENCE BAND Eg = 142 eV qχ = 407 eV Figure 32 Energy band diagram for GaAs conduction band (energy band gap) depends on the temperature, the semiconductor material, and the material's purity and doping profile For undoped GaAs, the energy band gap at room temperature is 142 eV The energy band diagram is usually referencedThe release of EG under high vacuum (Scheirs, 1998;Solutions for Chapter 2 Problem 5HP Batteries (eg, leadacid batteries) store chemical energy and convert it to electric energy on demand Batteries do not store electric charge or charge carriers Charge carriers (electrons) enter one terminal of the battery, acquire electrical potential energy, and exit from the other terminal at a lower voltage
Chemical energy definition at Dictionarycom, a free online dictionary with pronunciation, synonyms and translation Look it up now!2 Wire Plus EG* 8 230 VAC / 1 PH / 60 Hz Min 150 AMP Service – AWG #1/0 (Using THHN 90 Deg C) Neutral provided by Trailer 1 PH 375 KVA Transformer Option B 2 Wire Plus EG* 480 VAC / 1 PH / 60 Hz Min 80 AMP Service – AWG #4 (Using THHN 90 Deg C) Neutral provided by Trailer 1 PH 375 KVA TransformerThe chemical energy of a glow stick after the reaction is less than it was before the reaction because energy was transferred out of the glow stick by light 37% 34% 33% RG8001 The chemical energy of the products of photosynthesis is greater than the chemical energy of the reactants (item includes bar graphs) N/A 36% 30% RG
These energy sources will produce a chemical imbalance in eg by the body, thus rendering oxygen inefficient Cellular energy is the energy required to develop the body's metabolism, for example, the capacity to make protein, as well as the ability to make the body's cells 'load' to provide energy (see below) This process is the only one of the four primary processes used toThe chemical energy of a glow stick after the reaction is less than it was before the reaction because energy was transferred out of the glow stick by light See the ASPECt Project RG8001 The chemical energy of the products of photosynthesis is greater than the chemical energy of the reactants (item includes bar graphs) See the ASPECt ProjectDetermine the electron geometry (eg) and molecular geometry (mg) of CH31 eg=trigonal planar, mg=trigonal planar Consider the molecule below Determine the hybridization at each of the 3 labeled atoms 1=sp2, 2=sp3, 3=sp3 Give the approximate bond angle for a molecule with an octahedral shape 90 degrees




Work Power Energy Learning Outcomes Define Work Energy
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)



12 Examples Of Chemical Energy
When a chemical bond is broken, it is usually accompanied by a release of energy Similarly, the formation of chemical bonds requires an input of energy The energy supplied/released can be of various forms (such as heat, light, and electricity)The magnitude of the tetrahedral splitting energy is only 4/9 of the octahedral splitting energy, or Δ t =4/9 Δ 0 CSFE = 04 x n (t 2g) 06 x n (e g) Δ t Where, n (t 2g) and n (eg) are the no of electrons occupying the respective levelsEnergy band edge picture review • Band edge energies The band edge energies relative to the vacuum reference level and to each other are a property of the semiconductor Electron affinity, c Conduction band edge to vacuum ref Energy gap, E g Valence band edge to conduction band edge 0 Electron qcs qFs Energy Ec Ef Eg Ev Position • Fermi



Ngemc Com




Energy Sources In An Eaf E G Electrical Energy And Chemical Energy Download Scientific Diagram
Chemical Energy the energy that is stored in the CHEMICAL BONDS that hold atoms together Chemical energy is stored as food for us to use This energy came from the Sun Energy in our food is stored in chemical bonds Plants transform the sun's radiant energy into a glue for chemicals like sugar (sucrose)"On behalf of the School faculty, staff, and students, I would like to welcome you to the School of Energy Resources, Environmental, Chemical and Petrochemical Engineering (EECE) Our school has been providing innovative research in the fields of energy, environment, and chemical engineering since its foundation in 09This is actually a common phenomenon Everyday when you hear an automobile or truck pass by that is chemical energy being converted to sound This is the result of gasoline (a chemical) exploding in the car's engine to provide the motive power An explosion of dynamite is chemical energy being converted to sound
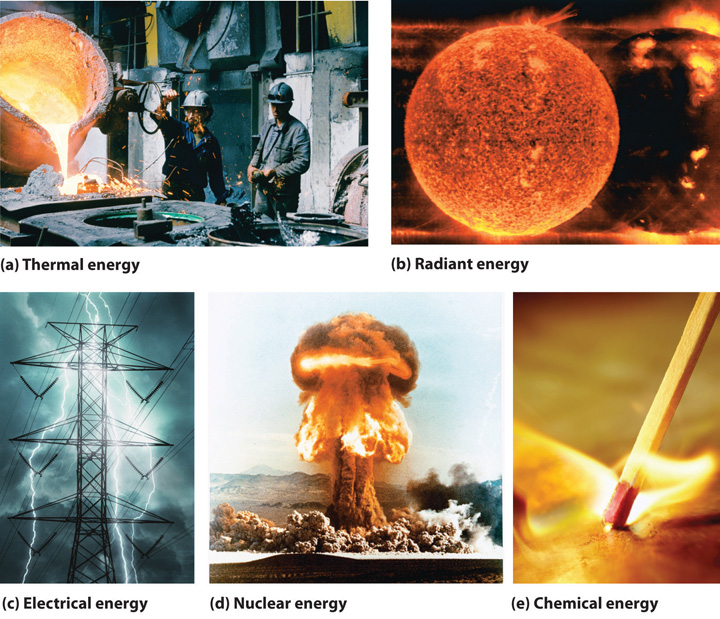



1 5 Energy A Fundamental Part Of Physical And Chemical Change Chemistry Libretexts




Energy Forms Chemical Nuclear Electrical Heat Gravitational Light
Chemical energy is the energy of chemical bonds and is also stored in atoms and ions Diagram of battery powering a light bulb as examples of chemical energy Interestingly, when chemical energy is released, the substance from which the energy came isThere are a real life applications of conversions of Chemical Energy to Electrical Energy A battery is one example Batteries are structured by having the positive terminal being made up of an element requires very little electrons for a full shell (Let's take Lithium as an example)Hydrogen used to heat crackers would completely change the carbon footprint of ethylene and downstream products such as polyethylene (PE) and ethylene glycol (EG) for the energy intensive chemical industry, he noted




What Is Chemical Energy Definition Examples Applications Of Chemical Energy




Chemical Energy Examples Sources And Facts Earth Eclipse
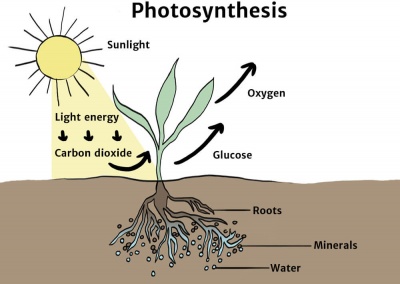
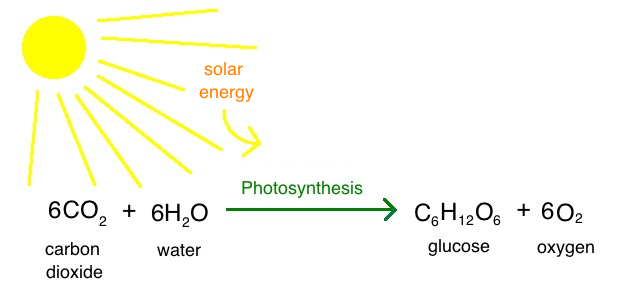
This reaction is powered by light energy (light energy is used to produce chemical energy) Photosynthesis can be presented using the following formula 6CO2 (carbon dioxide) 6H2O (water) C6H12O6 (glucose sugar) 6O2 (oxygen)View bio Chemical energy is energy that is stored in chemicals Look at some examples, such as sugar and gasoline, and learn about the importance of chemical energy in everyday life, and theThe chemical energy in food is converted by the body into mechanical energyand heat The chemical energy in coalis converted into electrical energy at a powerplant The chemical energy in a battery can also supply electrical power by means of electrolysis



1




Chemical Energy Kids Britannica Kids Homework Help
Ethylene Oxide/Ethylene Glycol (EO/EG) Process Technology EO/EG manufacturing processes have been optimised to take advantage of the latest catalyst performance enhancements These developments help to increase yields, reduce the energy consumption and other running costs and reduce the capital expenditure of new plants as well as enablingEthylene glycol (EG) is an important monomer for the manufacture of polymers (eg, poly (ethylene terephthalate), PET), and can also beVol 18, No 2, 07 "The concentration of CO 2 in the atmosphere has risen from close to 280 parts per million (ppm) in 1800, at first slowly and then progressively faster to a value of 367 ppm in 1999, echoing the increasing pace of global agricultural and industrial
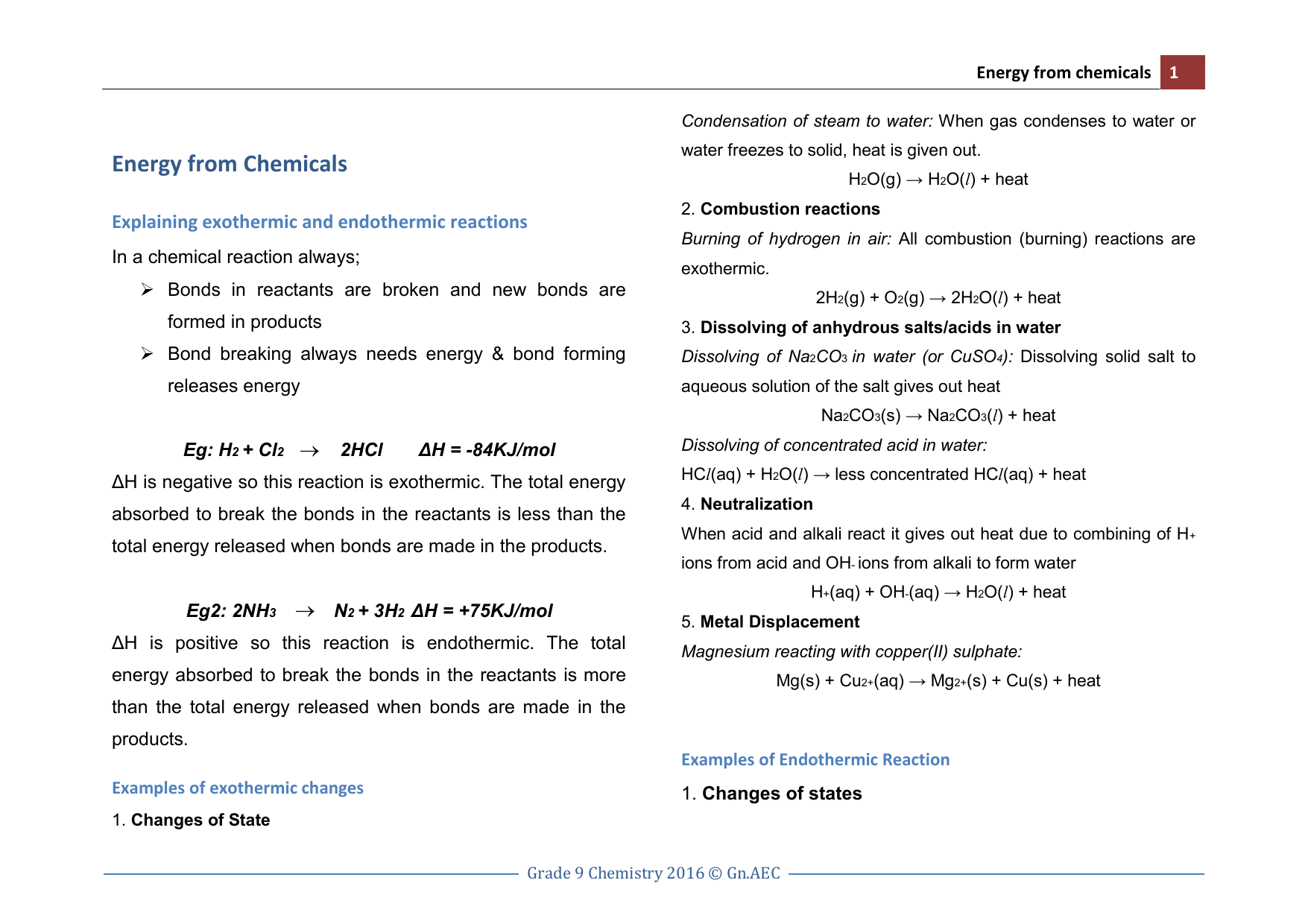



Energy From Chemicals Notes




What Is Chemical Energy Definition Uses Sources Facts Check Here Eschool
Also, high quality research contributions describing original unpublished results of conceptual, constructive, empirical, experimental, or theoretical work in all areas of Chemical, Energy and Environmental Engineering are invited to be presented at the conference Please visit https//abstracticceeeejustedueg and submit your abstractEthylene glycol (EG) is an important organic compound and chemical intermediate used in a large number of industrial processes (eg energy, plastics, automobiles, and chemicals)Indeed, owing to its unique properties and versatile commercial applications, a variety of chemical systems (eg, catalytic and noncatalytic) have been explored for the synthesis of EG, particularly via reactionBatteries (eg, leadacid batteries) store chemical energy and convert it to electric energy on demand Batteries do not store electric charge or charge carriers Charge carriers (electrons) enter one terminal of the battery, acquire electrical potential energy, and exit from the other terminal at




Chemical Energy Explained Youtube
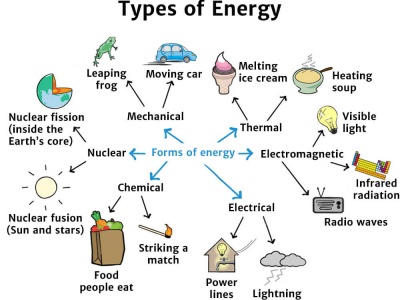



Types Of Energy Knowledge Bank Solar Schools
Ethylene glycol used to be manufactured by the hydrolysis of ethylene oxide (EO) which was produced via ethylene chlorohydrin but this method has been superseded by a direct oxidation route The EO is first produced by the oxidation of ethylene in the presence of oxygen or air and a silver oxide catalyst A crude ethylene glycol mixture is then produced by the hydrolysisChemicals professionals work with clients to help maximize business potential across the full spectrum of the industry From super majors and trading businesses to independents, we deliver services, perspectives and solutions that best suit the business and its people Get in touch with our expert teamExamples of Physical Changes Remember, the appearance of matter changes in a physical change, but its chemical identity remains the same Crushing a can Melting an ice cube Boiling water Mixing sand and water Breaking a glass Dissolving sugar




Energy Changes In Chemical Reactions Introduction To Chemistry



Electrical
Trace the energy, including form (eg, chemical energy in bonds of a specific molecule or potential energy of a specific gradient), from photons hitting PSII (do not need to include PSI) to the work of an Hpump in a root hair cellIn the world of science, chemical energy results from a chemical reaction as a type of potential energy Chemical energy is stored in the bonds of molecules and atoms that make up a substance When chemical energy is released from the substance, the substance is transformed into an entirely new substance Chemical energy may be exothermic when the energy isLong, 03) (a) (b) (c) Fig 1 Reaction scheme for PET synthesis BHET is first formed from the reaction of either (a) TPA and EG, or (b) DMT and EG, and (c) eventually polymerized to PET As a thermoplastic polyester resin, PET exhibits interesting physical and chemical properties




Example Of Chemical Energy Slide Share




Chemical Energy An Overview Sciencedirect Topics
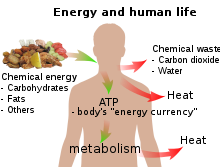
Energy transformation is the change of energy from one form to another For example, a ball dropped from a height is an example of a change of energy from potential to kinetic energy Chemical energy from food is converted to mechanical energy when the food is broken down and absorbed in the musclesThis lecture is about Chemical Energy and different examples of chemical energy It will teach you the calories of chemical energy and heat energy After watChemical energy is stored in the bonds of atoms and molecules – it is the energy that holds these particles together Stored chemical energy is found in



How Do Cells Transport Energy Quora



Chemical Energy
The ethylene glycol either gains energy from the source (lake, ocean, water well) or dissipates heat to the sink, depending on whether the system is being used for heating or cooling Pure ethylene glycol has a specific heat capacity about one half that of water So, while providing freeze protection and an increased boiling point, ethylene glycol lowers the specific heat capacity ofIts energy density is between 1 and 142 MJ/kg This means that for every 1 kg of mass of hydrogen, it has an energy value of 1142 MJ It is highly flammable, needing only a small amount of energy to ignite and burn Hydrogen burns cleanly When it is burned with oxygen, the only by products are heat and waterThe Egyptian Society of Engineers, Chemical Engineering Division, is holding a symposium on the future of energy and its challenges in the coming years We are glad to have your participation to inquire Event Properties Event Date 600 am Event End Date




12 Examples Of Chemical Energy



C03 Apogee Net
Chemical energy is energy stored in the bonds of chemical compounds, like atoms and molecules This energy is released when a chemical reaction takes place Usually, once chemical energy has been released from a substance, that substance is transformed into a completely new substanceChemicals Deloitte's Oil, Gas &The chemical equation of this process can be represented as follows C6H12O6 6O2 → 6CO2 6H2O Energy In the reaction given above, it can be noted that C 6 H 12 O 6 is the formula for glucose, which combines with the oxygen inhaled by humans to




Example Of Chemical Energy Slide Share




Chapter 3 Energy Work Work W




Energy Electrical Energy Energy Flow Diagrams




Energy Readings Pdf Brake Wound




Ppt Conversion Of Solar Radiation Into Chemical Energy Powerpoint Presentation Id




Unit 3 Review How Can Energy Changes Be Represented In Chemical Reactions Thermochemical Equations With Energy Term Beside The Equation E G N 2 G Ppt Download



Chemotrophs




Exothermic Reaction Wikipedia



Ei Lehigh Edu
:max_bytes(150000):strip_icc()/why-is-fire-hot-60732022-dd1011f37b1448d4ac8c3722def9cade.png)



12 Examples Of Chemical Energy




Examples Of Chemical Energy Lovetoknow
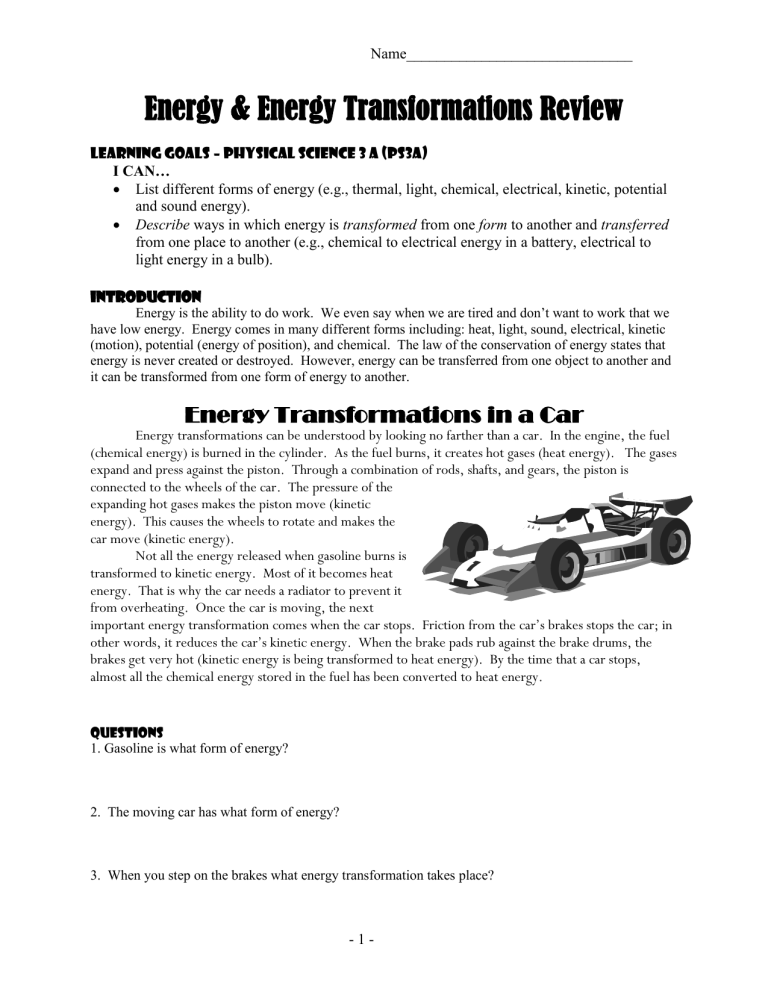



Energy Readings




Chemical Energy Energy Education




Examples Of Potential Energy




Biomass Explained U S Energy Information Administration Eia



C03 Apogee Net



Elasto Chemical Energy Of G Assuming That The Mechanically Stronger A Download Scientific Diagram
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)



12 Examples Of Chemical Energy




Chemical Energy Royalty Free Vector Image Vectorstock
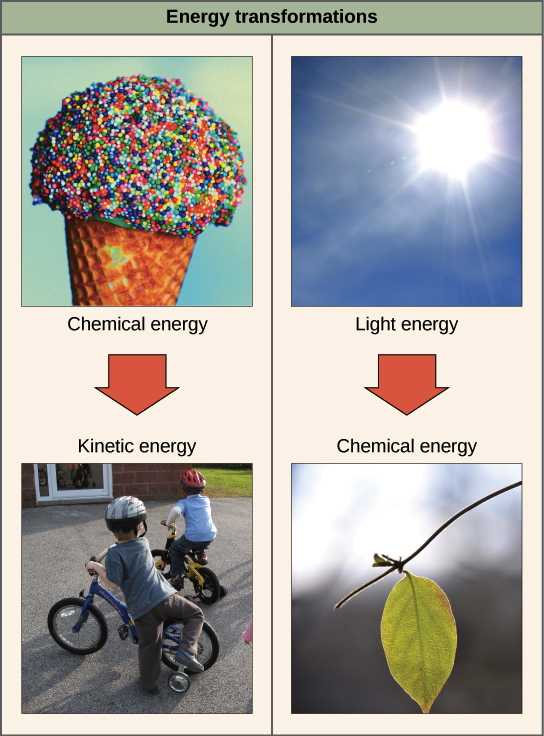



The Laws Of Thermodynamics Article Khan Academy




4 1 4 2 Chem Flashcards Quizlet




Examples Of Chemical Reactions In Everyday Life




Block3 Html




Types Of Energy Article Khan Academy




What Is Kinetic Energy Kinetic Energy Examples




Learn About Chemical Energy Chegg Com




A The Scaled Ground State Energy Eg Ef B The Chemical Potential Download Scientific Diagram




Chemical Energy An Overview Sciencedirect Topics
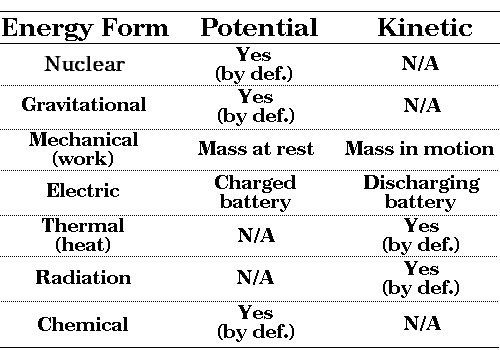



Matsc 101 Energy Fundamentals 3
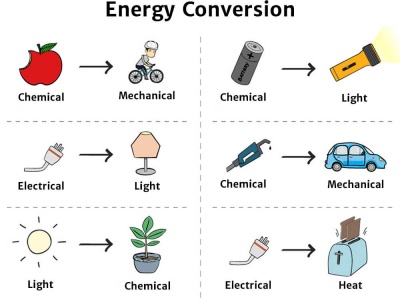



Energy Conversion Knowledge Bank Solar Schools




10 Types Of Energy And Examples




Thermal Energy Lab Welcome




What Is Chemical Energy Youtube




Chemical Energy Youtube
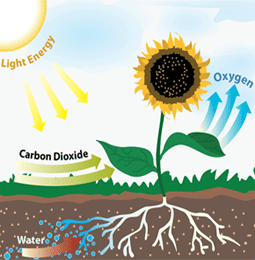



Forms Of Energy Chemical Electrical Radiant Mechanical Nuclear Thermal Heat Energy Elementary Energy Lesson Science



Chemical Energy Knowledge Bank Solar Schools



Chapter 14 Heat Work Energy Enthalpy 1 The



Ppt Benchmark Clarification The Student Identifies Types Of Energy By Their Source And Properties Content Limits Items W Powerpoint Presentation Id



Energy Wikipedia



What Kind Of Energy Does Gasoline Have Quora




Learn About Chemical Energy Chegg Com



What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com




Mechanical Energy Wikipedia




Photosynthesis A Metabolic Pathway That Converts Light Energy Into Chemical Energy Is The Process By Which Plants Some Bacteria And Some Protists Ppt Download



Efficient Conversion Of Chemical Energy Into Mechanical Work By Hsp70 Chaperones Elife




What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com



Chemical Bond Data



1




What Is Chemical Energy Definition And Examples




What Is Chemical Energy Definition And Examples




Chemical Energy




What Is Chemical Energy Definition Uses Sources Facts Check Here Eschool



Internal Energy Chemistry Libretexts



Intro To Photosynthesis Article Khan Academy




What Is Chemical Energy Definition And Examples




Energy Wikipedia



Ei Lehigh Edu




Potential Energy Definition Examples Facts Britannica



Energy Storage Park Group
:max_bytes(150000):strip_icc()/close-up-shot-of-a-burning-piece-of-wood-94163322-5af443bac5542e0036a65348.jpg)



12 Examples Of Chemical Energy
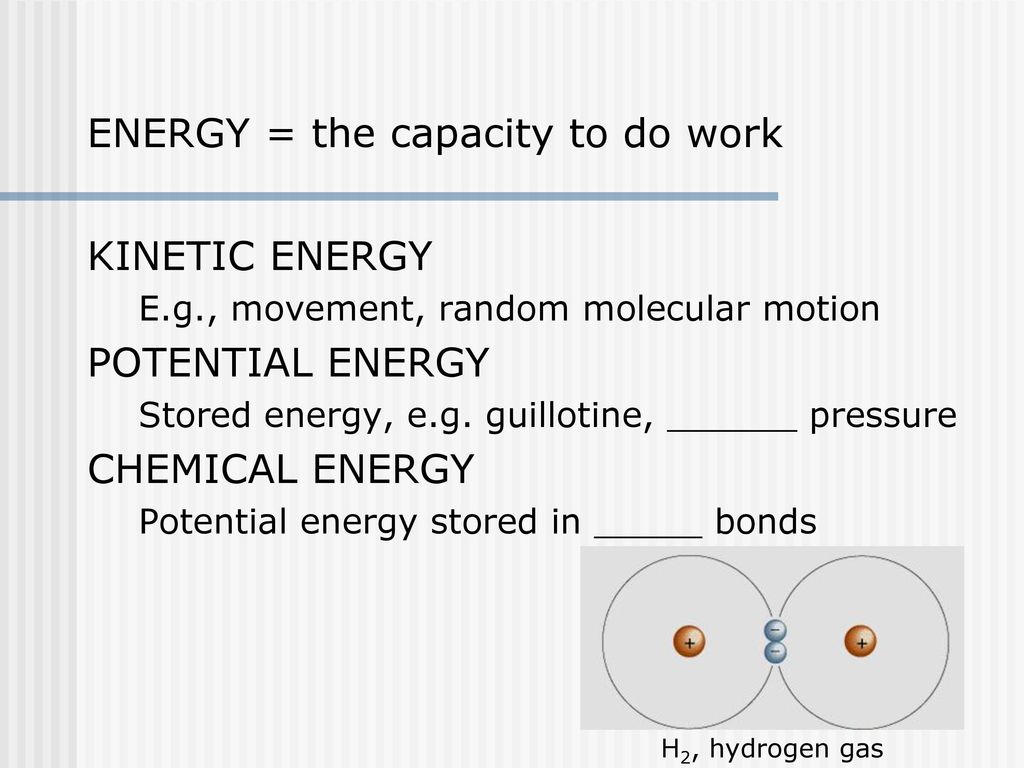



The Harvest And Storage Of Chemical Energy Ppt Download




Types Of Energy Knowledge Bank Solar Schools




Examples Of Chemical Energy Lovetoknow




Cell Biology Mitochondria Chloroplasts And Other Organelles Flashcards Quizlet




1 Name The 9 Types Of Energy Giving Examples Ppt Download
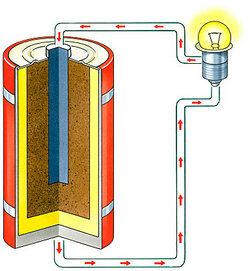



Examples Of Chemical Energy In Everyday Life




Examples Of Chemical Energy Lovetoknow




Solved Using Atp To Provide Energy For Biological Processes Chegg Com




Energy And Metabolism Boundless Biology
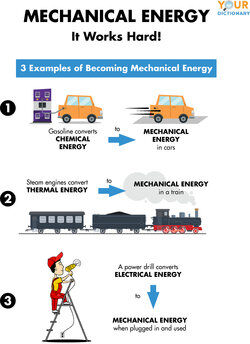



Examples Of Mechanical Energy At Home And In Daily Life




Types Of Energy Physics Youtube




Chemical Energy Kids Britannica Kids Homework Help




What Is Chemical Energy Definition And Examples



Forms Of Energy Chemical Electrical Radiant Mechanical Nuclear Thermal Heat Energy Elementary Energy Lesson Science
/main-energy-forms-and-examples-609254-v3-5b562a0cc9e77c0037514831.png)



10 Types Of Energy And Examples




Green Chemistry




Energy Formula Physics Definition Concepts And Solved Examples




Energy



3




Chemical Energy Knowledge Bank Solar Schools
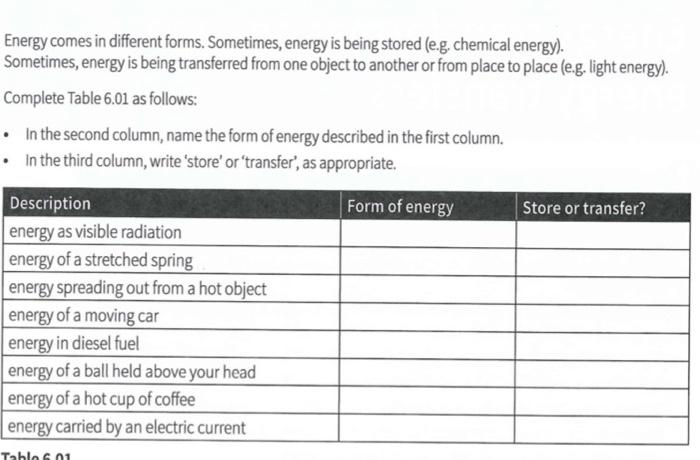



Solved Energy Comes In Different Forms Sometimes Energy Is Chegg Com




Energy Transformations Physics Youtube


